Why Real-World External Control Arms Are Gaining Ground in Health Technology Assessment
As payers and HTA bodies increasingly demand robust comparative evidence beyond what single-arm trials can provide, real-world external control arms (RW-ECAs) have become a pragmatic approach to bridge critical evidence gaps when randomized controlled trials (RCTs) are impractical or unethical. By leveraging observational cohorts from claims data, registries, or electronic health records, RW-ECAs can provide robust comparative insights to support health technology assessment (HTA) submissions. In this post, we explore the opportunities and challenges inherent in these designs, outline methodological best practices to withstand payer scrutiny, and highlight Putnam’s proven capabilities in delivering fit-for-purpose RW-ECA evidence.
What Are Real-World External Control Arms?
A real-world external control arm is an observational cohort derived from real-world data (RWD) that functions as a non-concurrent, non-randomized comparator for a single-arm trial. The foundation of any RW-ECA rests on four pillars:
- Fit-for-purpose data sources that capture relevant populations, exposures, outcomes, and confounders.
- Eligibility mirroring to replicate trial inclusion/exclusion criteria, wash-out windows, and run-in periods.
- Temporal alignment ensuring index dates and follow-up windows correspond to trial enrollment.
- Covariate harmonization through a common data model to guarantee consistent variable definitions across data sources.
Together, these pillars ensure that an RW-ECA emulates the design rigor of a randomized trial, bolstering the credibility and robustness of comparative effectiveness estimates derived from real-world data.
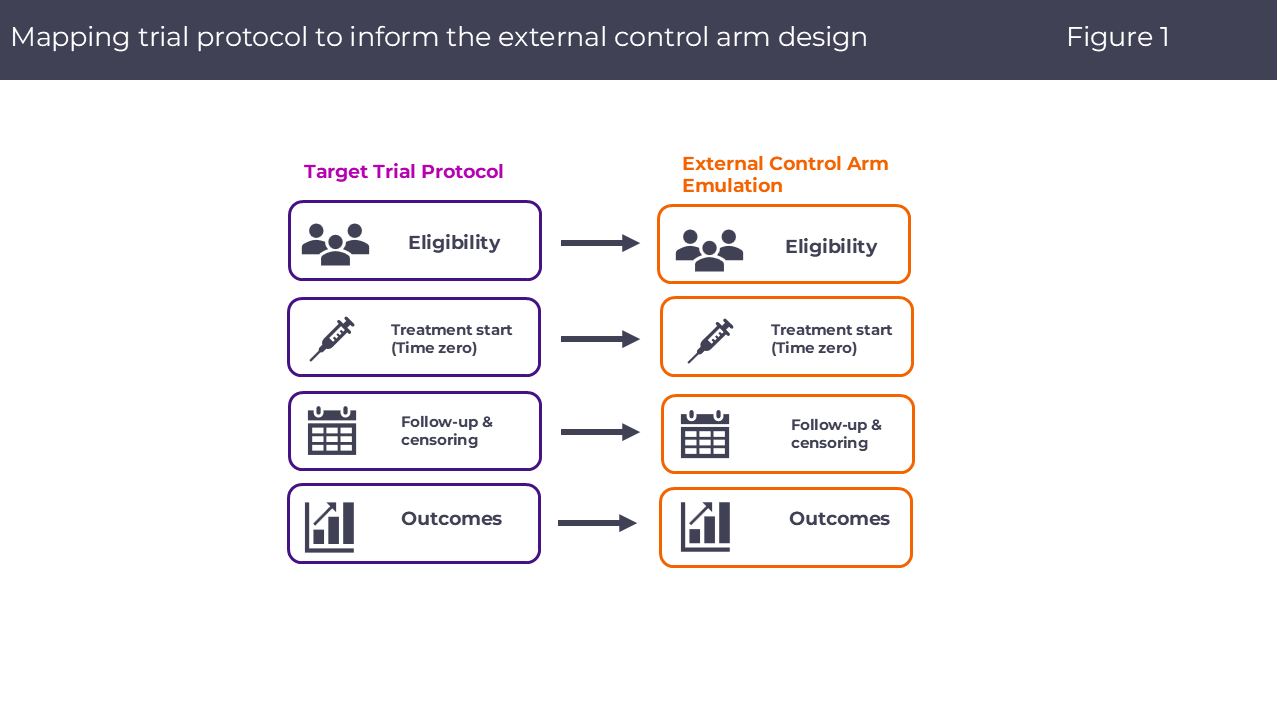
The Rising Role of RW-ECAs in HTA Submissions
Traditional RCTs remain the gold standard for HTA, but they pose significant hurdles in rare diseases, ultra-small pediatric populations, or scenarios where placebo-controlled designs may be deemed unethical. Single-arm trials are increasingly used as pivotal evidence in HTA submissions. Between 2019 and 2024, 18 NICE submissions incorporated RW-ECAs, 16 in oncology, one in cardiovascular, and one in rare disease settings. 1 Further, there has been a 20% increase in RW-ECAs submitted to global HTA agencies between 2018-2019 compared to 2015-2017, underscoring growing payer receptivity to these designs. 2
Methodological Best Practices for Designing Credible RW-ECAs
To withstand payer and regulatory scrutiny, RW-ECA designs should emulate a “target trial” framework:
- Specification of eligibility, treatment strategies, and index dates that mirror the single-arm trial.
- Follow-up and censoring rules aligned with trial protocols, with clear handling of discontinuation and competing risks.
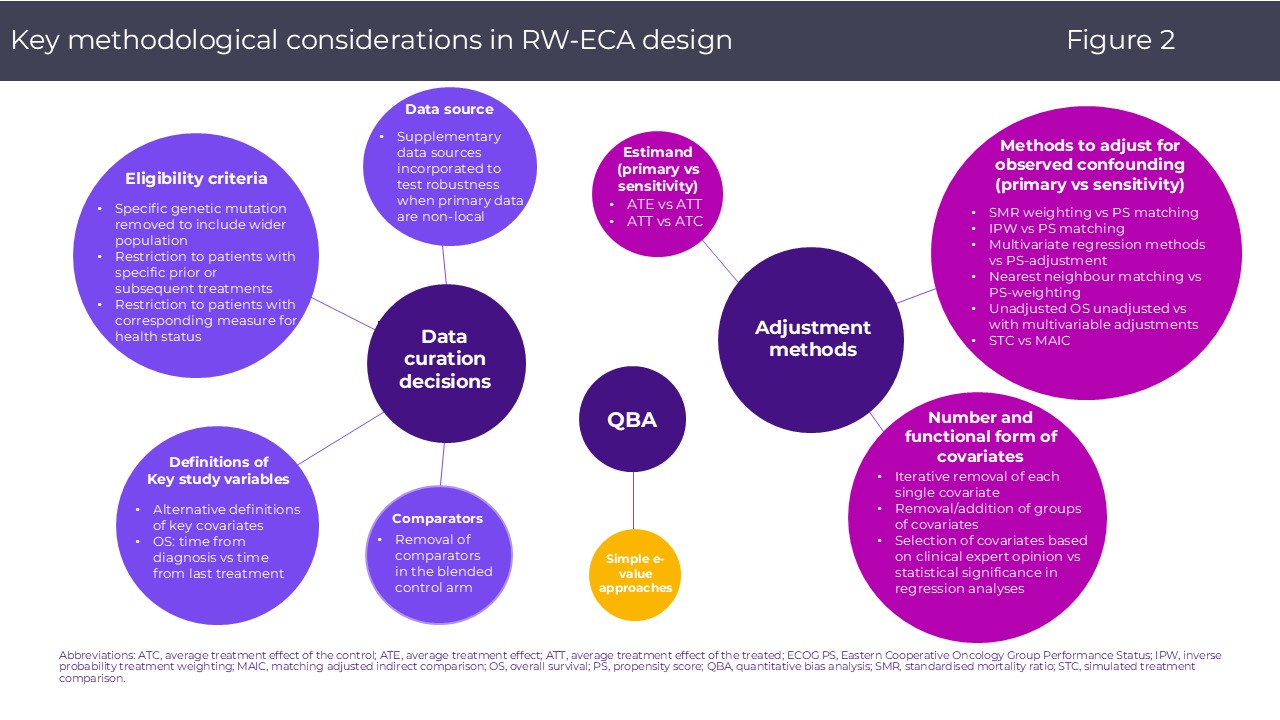
- Estimand clarity: Define primary (e.g., ATE vs. ATT vs. ATC) and sensitivity estimands, and pre-specify analytic contrasts.
- Adjustment for observed confounding: Employ propensity score methods (e.g., IPTW, matching) or augmented inverse probability weighting (e.g. AIPW), with prespecified covariate selection informed by clinical expertise.
- Quantitative bias analysis: Utilize E-value or tipping-point analyses to characterize residual uncertainty.
- Sensitivity analyses: Iteratively vary key parameters including covariate sets, exposure definitions, data sources, to demonstrate result robustness.
By implementing these elements in a transparent statistical analysis plan and reporting per accepted guidelines, sponsors can proactively address committee and evidence review group critiques and bolster the credibility of the RW-ECA evidence. The figure below illustrates key methodological considerations in RW-ECA design, spanning data curation, adjustment strategies, and quantitative bias analyses.
In a recent Putnam RW-ECA study led by our RWE & Biostatistics team, we emulated a target trial to compare outcomes from a single-arm olutasidenib trial against an external RWD cohort of patients receiving ivosidenib for relapsed or refractory modified IDH1 acute myeloid leukemia post-venetoclax.3 By applying entropy balancing, prespecifying all eligibility and follow-up criteria including estimands, and conducting sensitivity analyses, the study demonstrated how robust confounder control and transparent estimand specification can deliver credible comparative estimates even when sample sizes are constrained.
Opportunities and Challenges of RW-ECAs in Health Technology Assessment
Opportunities
- Accelerated approvals and label expansions: With 67 % of FDA accelerated approvals (1992–2017) based on single-arm trials,4 RW-ECAs can substantiate comparative claims when traditional RCTs are impractical.
- Informing rare disease decisions: In indications where sample sizes preclude well-powered RCTs, a RW-ECA can fill the comparative void, facilitating more informed HTA deliberations.
- Enhanced evidence depth: Multi-source analytics (claims + EMR) enable richer insights into patient subgroups, endpoints, and safety profiles.
- Global Evidence Re-use: Leverage a single RW-ECA across multiple HTA submissions by applying transportability re-weighting and hierarchical calibration to align diverse regional populations.
- Improved Reimbursement: Strengthen the value story during pricing negotiations by delivering more credible effect estimates, underpinned by target trial emulation, quantitative bias analysis, and early scientific advice.
Challenges
While RW-ECAs offer significant advantages, they also introduce unique hurdles that must be proactively managed:
- Residual confounding and bias: Without randomization, unobserved biases can undermine validity. In a recent review, evidence Review Groups flagged residual confounding in nearly half of NICE submissions, often criticizing inconsistencies in eligibility criteria or lack of sensitivity analyses.1
- Heterogeneous endpoint definitions: Variability in how outcomes are captured (e.g., lab‐based response criteria versus structured clinical assessments) can complicate direct comparisons unless harmonized through a common data model and carefully aligned definitions.
- Data completeness and quality: Missing key variables and differential follow-up can all undermine analytic robustness without systematic data curation and transparent handling of missingness.
- Temporal and secular trends: Changes in standard‐of‐care over time or delays between trial enrolment and registry data collection can introduce bias if the external cohort isn’t contemporaneous.
- Transportability and external validity: Differences in patient characteristics, care settings, and temporal context between the trial and real-world cohorts can bias comparative estimates if not rigorously aligned and adjusted.
By recognizing these challenges up front and embedding fit-for-purpose mitigation strategies in the study protocol and analysis plan, a well-specified RW-ECA, can accelerate regulatory and HTA timelines, support label expansions, and ultimately expedite patient access to breakthrough therapies.
Conclusion
Real-world external control arms represent a strategic imperative for pharmaceutical sponsors navigating the complexities of HTA in rare or single-arm trial settings. By adhering to a robust target trial emulation framework, implementing quantitative bias analyses, and implementing a mitigation strategy for the possible analysis challenges, compelling, payer-ready evidence that accelerates patient access can be generated.
At Putnam, we partner with clients to translate methodological best practices into operational success, delivering evidence that not only meets but anticipates HTA expectations.
Our RWE & Biostatistics team will also be showcasing a recent related study at ISPOR Europe 2025 in Glasgow. Our abstract, ‘Influence of NICE Real-World Evidence Framework on HTA Submissions: Sensitivity Analyses in Real-World External Control Arm Studies,’ has been accepted as a Top 5% poster presentation. This recognition underscores the team’s leading role in advancing best practices for RW-ECAs and highlights our deep expertise in applying sensitivity analyses and quantitative bias analysis to address payer and regulator concerns. To explore how we can support your next RW-ECA submission, please reach out to our RWE & Biostatistics team contact us.
References:
- Leahy, T., Keenan, C., Robertshaw, E., McStravick, M., Sammon, C., & Turner, A. (2024). P2 exploring the unknown: Use of sensitivity analysis to characterize residual uncertainty in real-world external control arm evidence submitted to NICE. Value in Health, 27(12), S1.
- Patel, D., Grimson, F., Mihaylova, E., Wagner, P., Warren, J., van Engen, A., & Kim, J. (2021). Use of external comparators for health technology assessment submissions based on single-arm trials. Value in Health, 24(8), 1118–1125. https://doi.org/10.1016/j.jval.2021.03.005
- Lai, C. E., Leahy, T. P., Turner, A. J., Thomassen, A., Wang, L., Sheppard, A. D., & Cortes, J. (2025). Effectiveness of olutasidenib versus ivosidenib in patients with mutated isocitrate dehydrogenase 1 acute myeloid leukemia who are relapsed or refractory to venetoclax: The 2102-HEM-101 trial versus a US electronic health record-based external control arm. Leukemia & Lymphoma. Advance online publication.
- Beaver, J. A., Howie, L. J., Pelosof, L., Kim, T., Liu, J., Goldberg, K. B., … & Kluetz, P. G. (2018). A 25-year experience of US Food and Drug Administration accelerated approval of malignant hematology and oncology drugs and biologics: A review. JAMA Oncology, 4(6), 849–856. https://doi.org/10.1001/jamaoncol.2017.5618

Jump to a slide with the slide dots.
 Mariia Dronova
Mariia Dronova
Value of Equity in HTA: Current Guidelines and What to Expect
Equity in HTA is evolving. Explore current guidelines, future trends, and how Putnam advances equity methods in health assessments.
Read more Yemi Oluboyede
Yemi Oluboyede
Unlocking Patient Insights in HEOR Through Netnography: A Scalable Qualitative Research Method
In pharma commercialization, AI and analogs set the stage, but human insight drives strategies that break precedent and win markets
Read more Mariah Hanley
Mariah Hanley
AI as the Input, Not the Answer: When Human Strategy Still Leads
In pharma commercialization, AI and analogs set the stage, but human insight drives strategies that break precedent and win markets
Read more

